Bienvenue sur le site d'IPGP
Thèmes de recherche
Actualités
Chiffres clés

100
ans de sciences pour la planète

4
volcans actifs français surveillés par l'IPGP

520
membres du personnel (chercheurs, ingénieurs, techniciens, agents administratifs, post-doctorants et doctorants)

1
observatoire sur la planète Mars

512
publications en 2021 (dont 25 dans des revues à fort impact telles que Nature, Science et PNAS)

7
sites : Guadeloupe, Martinique, La Réunion, Chambon-la-Forêt, Champs-sur-Marne et 2 sites à Paris
Agenda
19 avril
Soutenance d'Habilitation à Diriger des Recherches
Nouveaux traceurs isotopiques pour décrypter les signaux géochimiques des archives sédimentaires et des volcans d’arc
Orateurs : Magali Bonifacie
23 avril
Séminaires thème Risques naturels
--- GRAND SEMINAIRE DE THEME --- Earthquake Physics: Bridging Tectonics and Dynamic Rupture in Space and Time
Orateurs : Luca Dal Zilio
24 avril
Séminaires Dynamique des fluides géologiques
Geomagnetism and numerical models: one way to get insights on the Earth’s outer core
Orateurs : Olivier Barrois
25 avril
Séminaires thème Intérieurs de la Terre et des planètes
Models of Mercury's interior evolution: Successes and challenges
Orateurs : Nicola Tosi
Dernières publications
Kawamoto Tatsuhiko, Inukai Toshihiro, Nicollet Christian, Koga Kenneth T., Rose-Koga Estelle F., Debret Baptiste. Fluid inclusions of ophicarbonates in Oman and Western Alps ophiolite and carbonation experiments of mantle minerals. 25 août 2024.
Peruzzetto Marc, Colas Bastien, Lévy Clara, Rohmer Jérémy, Bourrier Franck. Empirical quantification of rockfall reach probability: objective determination of appropriate topographic descriptor. 08 juillet 2024.
Zheng Kun, Benedetti Marc, Jain Rohan, Pollmann Katrin, van Hullebusch Eric. Recovery of gallium (and indium) from spent LEDs: Strong acids leaching versus selective leaching by siderophore desferrioxamine E. Separation and Purification Technology, Elsevier, juin 2024, 338. <10.1016/j.seppur.2024.126566>
Gaudaré Louis, Doubre Cécile, Jolivet Marc, Dauteuil Olivier, Corgne Samuel, Grandin Raphaël, Doin Marie-Pierre, Durand Philippe. Unraveling Tectonic From Hydrological Subsidence Of The Okavango Graben (Botswana) Using FLATSIM InSAR Data.. 14 avril 2024. <10.5194/egusphere-egu24-20218>
Nos observatoires
et stations

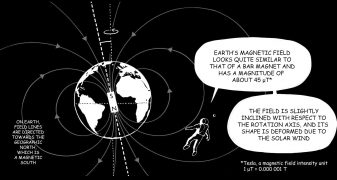
Mars
Service national d'observation InSight

La mission NASA InSight a déployé fin 2018 un observatoire géophysique à la surface de la planète Mars, réalisant des mesures sismiques, géodésiques et magnétiques simultanées. Ce service national d'observation est porté par l'IPGP, en collaboration avec plusieurs laboratoires et observatoires français (en particulier le LPG et GEOAZUR).
En savoir plus